Celthy Liposome Transfection Reagent, Confidence in Transfection

Background Introduction
Transfection enables us to better understand how target genes are expressed and regulated in cells. Common transfection methods currently include calcium phosphate co-precipitation, electroporation, DEAE-dextran and polybrene, mechanical methods (such as microinjection and gene gun), and cationic liposome transfection reagents, among which cationic liposome transfection reagents are commonly used.
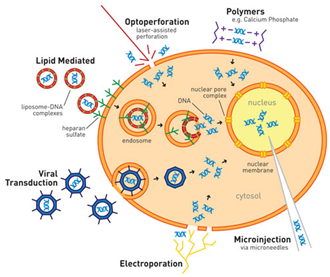
Figure 1: Schematic Diagram of Different Transfection Methods (Image source: Daily Biology Reviews)
Comparison of Different Transfection Methods
|
Transfection Method |
Advantages |
Disadvantages |
|
Calcium Phosphate Co-precipitation |
Low cost, relatively simple operation |
Unstable transfection efficiency, prone to issues such as DNA aggregation affecting transfection efficiency, high cytotoxicity, poor transfection efficiency |
|
Electroporation |
High efficiency, suitable for all types of cells |
Expensive electroporation equipment, high cell death rate, requires large amounts of nucleic acids and cells |
|
DEAE-Dextran |
Effective for transfection of adherent cells, can also be used for certain suspension cells, simple operation, repeatable results |
Cell preference, some toxicity to cells, serum inhibition of cell growth required during transfection |
|
Polybrene |
Relatively simple operation, moderate price |
Low efficiency, some limitations in application |
|
Cationic Lipid Reagents |
Simple operation, high efficiency, wide applicability, good repeatability, low toxicity |
Slightly higher price compared to other chemical reagents |
|
Viral Transduction |
High efficiency, effective for difficult-to-transfect cells, good transduction in primary cells |
Complex viral packaging process, some risk associated with viruses |
|
Polyethylenimine (PEI) |
Low price, simple operation, wide applicability |
Lower efficiency compared to lipid-based transfection reagents, some cells exhibit sensitive reactions after contact |
Celthy Trans liposome nucleic acid transfection reagent, as a cationic lipid transfection reagent, has higher transfection efficiency and more convenient operation compared to the widely used Lipo2000. It has been validated in various cell lines, as summarized in the list of validated cell lines below.
Mechanism of Action
Cationic lipids can wrap nucleic acids through electrostatic interactions due to the positive charges on their surface, forming nucleic acid-liposome complexes. The cell membrane surface carries a negative charge, allowing the complex to be adsorbed. Once adsorbed, the complex can enter the cell through membrane fusion or endocytosis, forming inclusion bodies within the cell. A small portion of DNA can be released from the inclusion bodies and enter the cytoplasm, further entering the cell nucleus for transcription and expression.
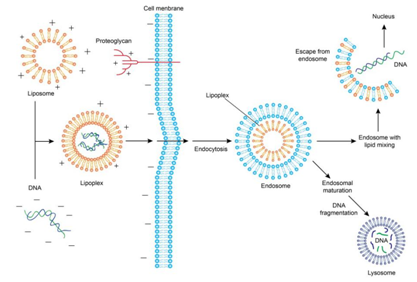
Figure 2: Liposome-mediated Transfection and Endocytosis Process
Celthy Trans Transfection Reagent Product Features
High efficiency: capable of transfecting most eukaryotic cells, with transfection efficiency exceeding 90% for common cell lines;
Low toxicity: transfected cells exhibit good morphology and express high levels of transfected gene proteins;
Convenient operation: liposome complexes can be directly added to serum-containing culture media;
Versatility: suitable for both transient and stable transfection experiments.
Operation Procedure
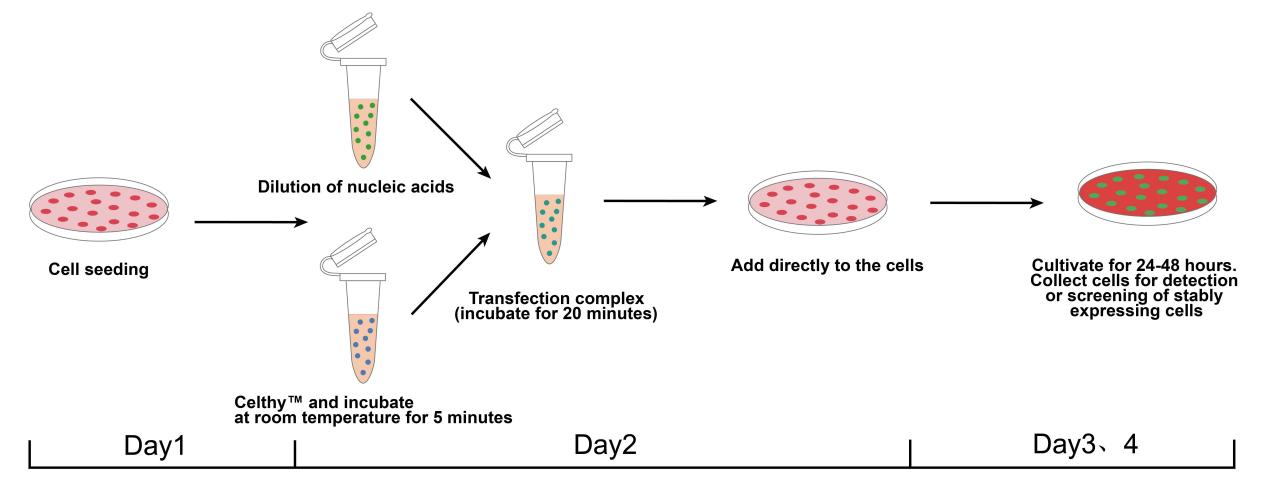
Figure 3: Cell Transfection Experimental Workflow
Product Data
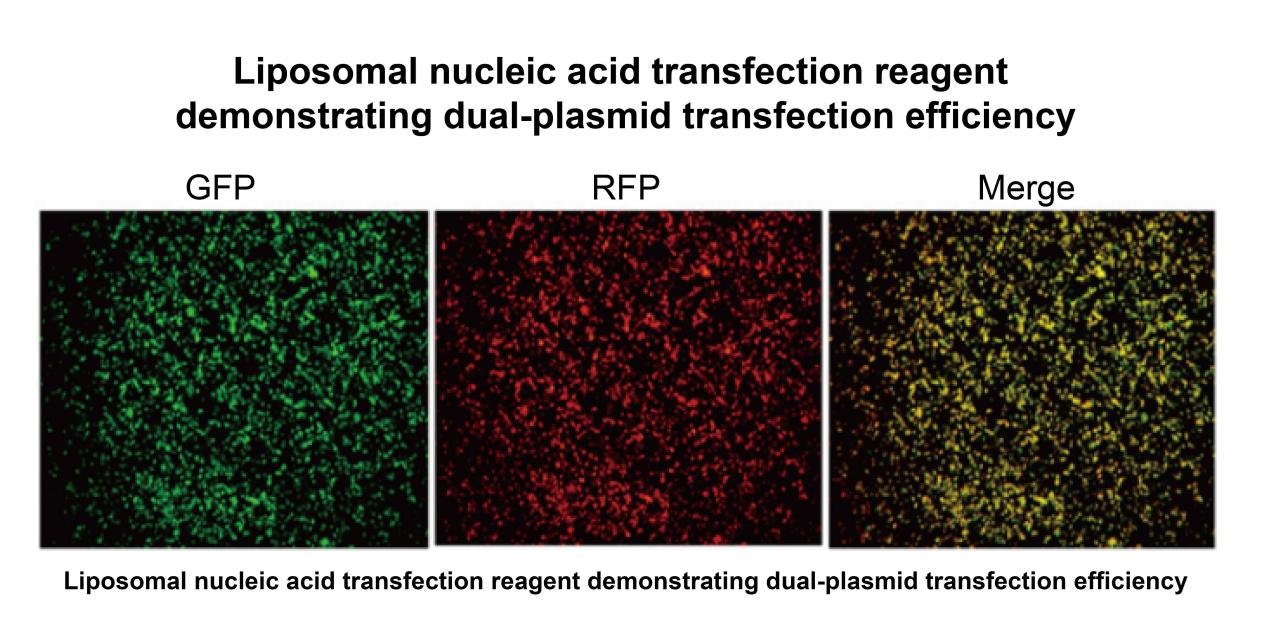
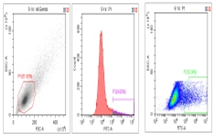
Figure 4: Dual Plasmid Transfection Reagent Validation Results
Summary of Validated Cell Lines
|
293T |
293FT |
HEK293 |
HEK 293T |
Caco2 |
|
COS-7 |
DF-1 |
Lenti X-293T |
HeLa |
HepG2 |
|
LM3 |
MCF-7 |
MDA-MB-231 |
MEF |
NCI-H1975 |
|
NIH-3T3 |
PC12 |
Raw264.7 |
Vero |
H9c2 |
|
Hepa1-6 |
MCF10A |
CHO |
5-8F |
BV-2 |
|
A549 |
3t3 |
H520 |
HUVEC |
C6 |
|
HaCaT |
N2A |
SGC-7901 |
H9 |
MKN-28 |
|
Hep 3B |
SMCC7721 |
RKO |
C2C12 |
More… |
Customer Feedback Results Presentation
Celthy Trans Liposomal Transfection Application
|
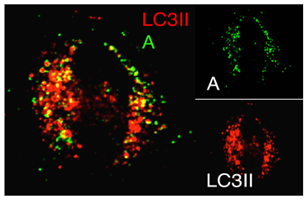
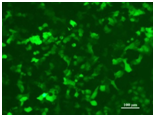
Activation of Cell Autophagosome Formation by Bacterial Protein Cell: HeLa Transfected Plasmid: Bacterial Effector Protein A LC3II: Autophagosome Marker Protein |
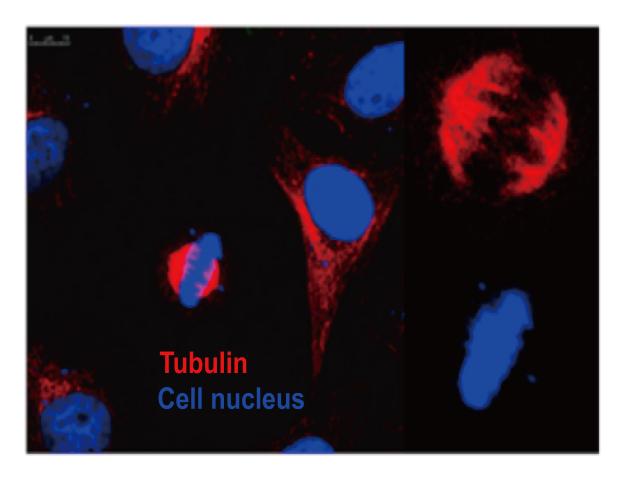
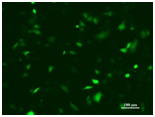
Hoechst 33342 Spindle Structure during Cell Division Cell: HeLa Transfected Plasmid: Flag-tubulin Nuclear Staining: Hoechst 33342 |
||
|
Celthy Trans |
Lipo3000 |
Celthy Trans |
Lipo2000 |
|
Image Source: Fudan University Cell Line: HEK293 Nucleic Acid Type: DNA |
Image Source: Changchun Institute of Applied Chemistry Cell Line: HeLa Nucleic Acid Type: DNA |
||
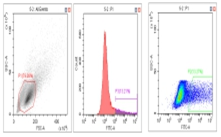
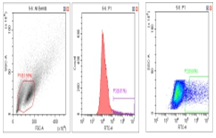
Flow Cytometry Fluorescence Observation Results
|
Celthy Trans (13.27%) |
Lipo2000 (8.64%) |
FuGenHD (9.24%) |
|
Cell Line: C2C12 Fluorescent Protein: EGFP (Enhanced Green Fluorescent Protein) Nucleic Acid Type: DNA Data Source: South China Agricultural University |
||
Partial Cell Transfection Data Provided by the Customer (For Reference Only)
|
Cell |
Culture Plate |
Cell Density |
DNA |
Celthy Trans |
Efficiency |
|
293T |
6 well |
80% |
1μg |
2μL |
90% |
|
293FT |
24 well |
85% |
1μg |
4μL |
90% |
|
Hela |
12 well |
90% |
0.2μg |
0.6μL |
90% |
|
Raw264.7 |
35mm plate |
80% |
1μg |
2μL |
90% |
|
Hek293 |
6 well |
95% |
2μg |
10μL |
80-90% |
|
NCI-H1975 |
6 well |
80% |
4μg |
10μL |
80% |
|
3t3 |
12 well |
90% |
1μg |
5μL |
50% |
FAQ
Q1: Can serum be present when preparing the nucleic acid transfection reagent complex?
a: The presence of serum can affect the formation of liposomes. It is recommended to use serum-free medium (usually MEM medium) when preparing the nucleic acid transfection reagent complex.
Q2: Is it necessary to change to serum-free medium during the transfection experiment?
a: No, our transfection reagent can be used for transfection in media containing serum.
Q3: Is it necessary to terminate the transfection after transfection?
a: No. The liposome complex can remain stable for 6 hours. If the cell medium has not been changed before transfection, it is necessary to change to fresh medium after 4-6 hours to ensure the nutrients required for normal cell growth. However, if the medium has been changed before transfection, it is not necessary to change the medium again after liposome transfection.
Q4: Precautions for storing and using the transfection reagent?
a: This reagent must be stored at 4°C. Avoid repeated long-term opening of the bottle, as prolonged exposure to air can cause oxidation of the liposomes and affect transfection efficiency.
Q5: What should be noted to improve transfection efficiency?
a: Cell density during transfection, 90%-95%.
b: Use MEM serum-free medium for dilution of nucleic acids and liposome diluents during transfection.
c: Medium change can be performed 4-6 hours after transfection.
Related Products
|
Product Name |
Catalog number |
Specification |
|
C130002S |
0.5 mL |
|
|
C130002M |
1.0 mL |
|
Application Scenario |
Product Name |
Catalog number |
Specification |
|
Cell Type: Adherent/Suspension Nucleic Acid Type: DNA, siRNA |
C130002S |
0.5 mL |
|
|
C130002M |
1.0 mL |
||
|
Cell Type: Adherent/Suspension Nucleic Acid Type: miRNA, siRNA,DNA |
C130003S |
0.5 mL |
|
|
C130003M |
1 mL |