How to Prepare Human Intestinal Organoids

The intestine is an important digestive organ in the human body, referring to the digestive tract from the pylorus of the stomach to the anus, and is the longest digestive organ in the body. The human intestinal structure mainly includes the small intestine and the large intestine. Intestinal diseases are very common, such as intestinal obstruction, chronic inflammatory bowel disease, colorectal cancer, etc., among which colorectal cancer has become one of the most serious diseases threatening human health. Therefore, research on the intestine is a very hot field with broad application prospects.
Cultivation Scheme - Literature Analysis 1
In 2009, the research team led by Hans Clevers successfully cultured 3D intestinal "organoids" in vitro for the first time using single stem cells (Lgr5+) or single crypt structures derived from intestinal crypts. Isolated intestinal stem cells or crypts can continuously proliferate in matrix gel, small molecular compounds, cell additives, and various growth factors, possessing self-renewal and differentiation capabilities. They can differentiate into various mature intestinal cells and play an important role in establishing and studying intestinal disease models. [1]
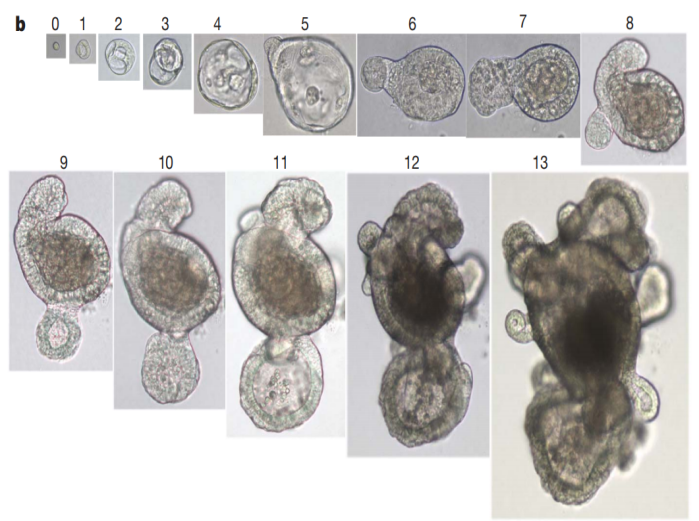
Figure 1. Efficiency of Single-Cell Colony Formation in Single Wells
1. Crypt Isolation, Cell Dissociation, and Cell Culture
Crypts are typically released from the mouse small intestine by incubating in PBS containing 2 mM EDTA at 4°C for 30 min. The isolated crypts are then counted and formed into spheres. In a 24-well plate, matrix gel and growth factors (10-50 ng/mL EGF, 500 ng/mL R-spondin 1, 100 ng/mL Noggin) are added for incubation.
2. Sorting Experiment
Isolated crypts are incubated at 37°C for 45 min, and the dissociated cells are passed through a 20 μm cell filter. The sorted cells are collected in crypt culture medium and added to matrix gel containing Jagged-1 peptide (1 μM), with one cell per well (5 mL matrix gel in a 96-well plate).
3. Crypt Culture Medium
250 μL of culture medium is added to a 48-well plate, and 100 μL of culture medium is added to a 96-well plate, supplemented with the small molecule Y-27632 (10 μM). Growth factors are added every other day, and the culture medium is changed every 4 days.
4. Passaging
Organoids are removed from the matrix gel, mechanically dissociated into single crypt structures, and then transferred to fresh matrix gel. Passaging is performed every 1-2 weeks, with a splitting ratio of 1:5. [1]
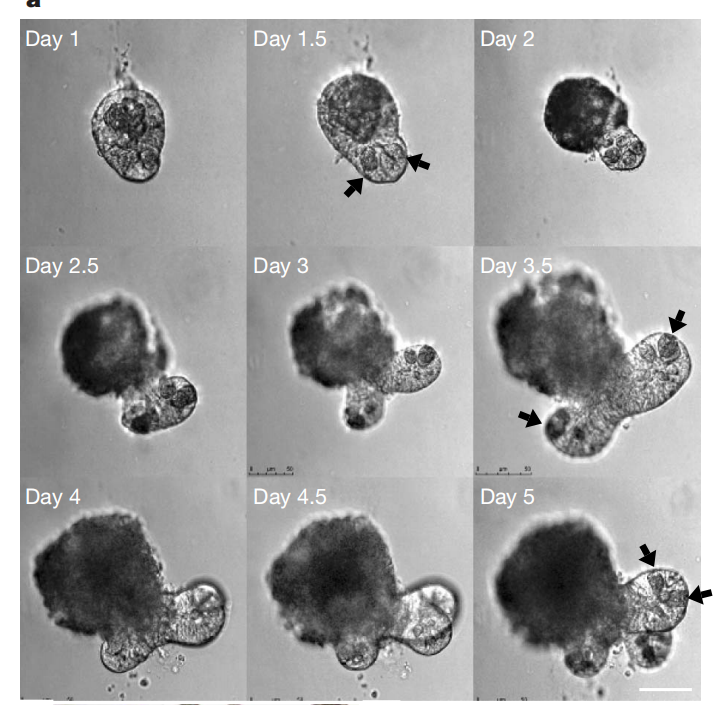
Figure 2. Establishment of Intestinal Organoid Culture System [1]
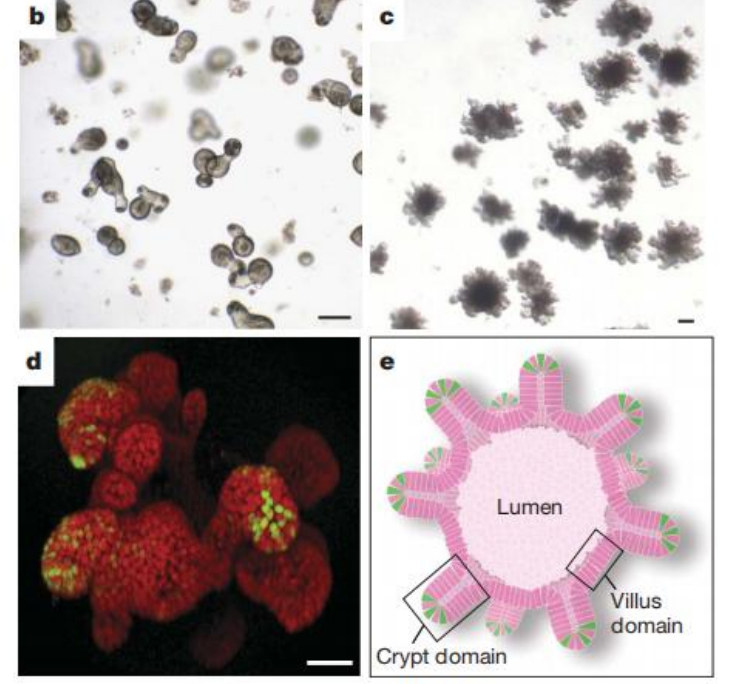
Figure 3. b, c, d depict the situations after 5 days, 14 days, and 3 weeks of cultivation respectively, while e represents a schematic diagram of crypt-like organoids.
When culturing mouse colon organoids, the culture medium needs to be supplemented with small molecule compounds including p38 MAPK kinase inhibitor (SB-202190), TGF-β inhibitor (A 83-01), Rho kinase inhibitor (Y-27632), GSK inhibitor (CHIR-99021), Jagged-1, as well as growth factors such as EGF, R-spondin-1, Noggin, and Wnt-3A. [1] [2]
The research team designed such a culture system based on the growth requirements of intestinal epithelial cells. Wnt signaling pathway is crucial for crypt proliferation, and the Wnt agonist R-spondin1 induces significant crypt hyperplasia in vivo. The signaling transduction of epidermal growth factor (EGF) is associated with intestinal proliferation, while transgenic expression of Noggin induces an increase in crypt number. Rho kinase inhibitor Y-27632 suppresses embryonic stem cell apoptosis and reduces cell death rates. Notch signaling between cells is essential for maintaining crypt proliferation, and we also provide a Notch agonistic peptide (Jagged-1). [1] [2]
Literature Analysis 2
In 2022, the Hans Clevers team innovatively introduced IL-22 to optimize the composition of the hSIOs culture medium, significantly increasing the budding ratio of intestinal organoids and upregulating the expression levels of Paneth cells, better simulating the compositional expression components of the human intestine, as shown in the figure below.
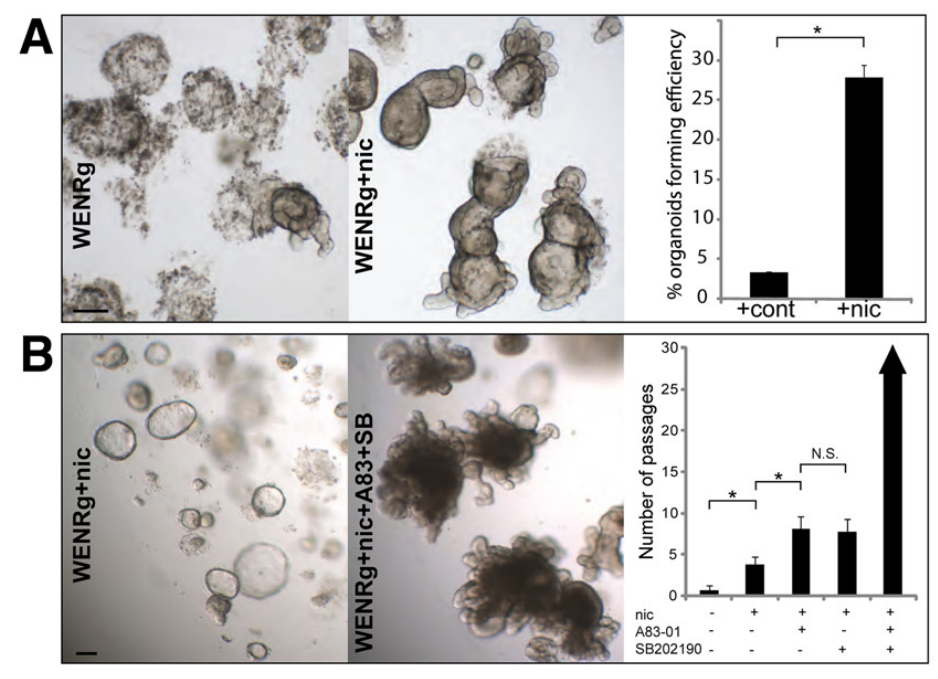
Figure 4. Cultivation of human colon (effects of small molecules Nicotinamide, SB 202190, and A 83-01 on cultivation)
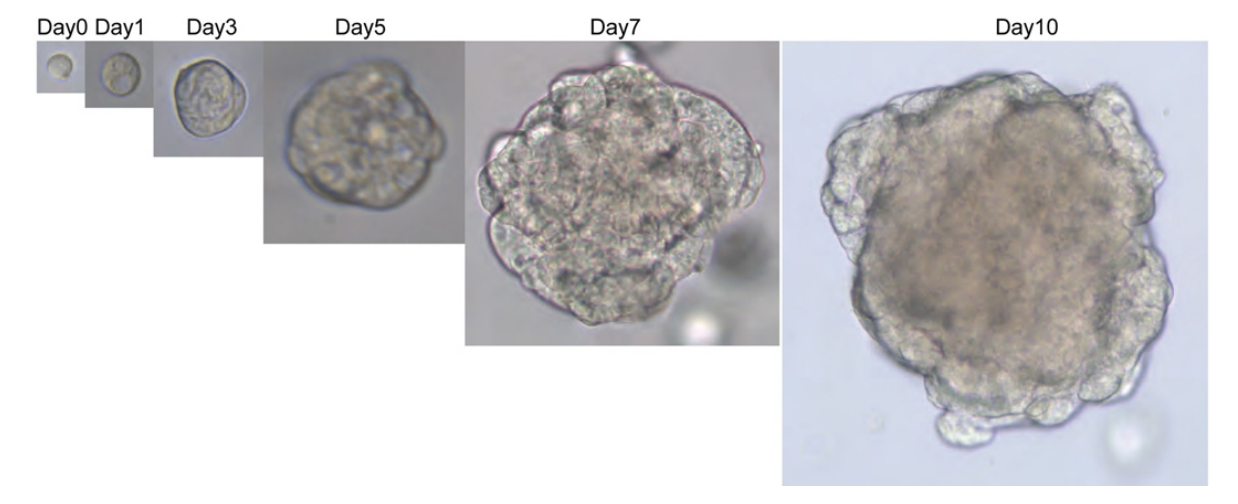
Figure 5. Cultivation process of human colon adenocarcinoma cells
Literature Analysis 3
The small molecule compounds, additives, and cytokines required in the culture medium for organoid cultivation and drug stimulation of hSIOs include: B-27 (1%), N-acetylcysteine (1 mM), niacinamide (10 mM), Alk 4/5/7 inhibitor A 83-01 (500 nM), p38 inhibitor SB 202190 (3-10 μM), prostaglandin E2 (10 nM), CHIR-99021 (3-10 μM), Rho kinase inhibitor Y-27632 dihydrochloride (10 μM), and Primocin (100 μg/mL), BP-1-102, LY294002, MK-2206, rapamycin, N-2, glutamine, HEPES, penicillin/streptomycin, R-Spondin, Noggin (2%), human EGF (50 ng/mL), human IL-22 (2 ng/mL), as specifically illustrated in Figure 6. [3]
|
Compound Name |
Product Number |
|
Recombinant Human IL-22 Protein |
C230399 |
|
Recombinant Mouse Interleukin-22 (Mouse IL-22) |
C230571 |
|
Recombinant Human Wnt -3a Protein |
C230259 |
|
Recombinant Human Noggin Protein,His Tag |
C230462 |
|
Recombinant Human EGF Protein,His Tag |
C230329 |
Cultivation Steps for Human Intestinal Organoids
1. Reconstitute the crypt matrix gel mixture and add a certain amount of the mixture to the well plate along with intestinal organoid culture medium (e.g., for a 24-well plate, add 50 μL of mixture and 500 μL of culture medium).
2. Transfer to a laminar flow hood, rinse 2-3 times with PBS (containing antibiotics), transfer the intestinal mucosa to a solution containing EDTA (2 mM), and incubate at 4°C for approximately 30 min for small intestine digestion and approximately 1 hour for colon digestion.
3. Transfer the digested small intestinal mucosa to a centrifuge tube, add PBS, shake well, filter through a 70 μm cell strainer, centrifuge at 1000 rpm at room temperature for 5 min. Colon crypts do not need to be filtered.
4. Discard the supernatant, add culture medium to resuspend the pellet, count the crypts, with an optimal quantity of approximately 10 crypts/μL.
5. Add a certain amount of suspension to a centrifuge tube and centrifuge at room temperature for 5 min.
6. Add pre-chilled matrix gel mixture (matrix gel: culture medium = 2:1) to resuspend the pellet.
7. Inoculate the resuspended pellet in preheated well plates according to a certain quantity.
8. Add a certain amount of preheated organoid culture medium to each well, and culture at 37°C for 1-2 weeks.
Passaging of Human Intestinal Organoids:
9. Add a certain amount of culture medium to each well in the culture plate, carefully scrape out the matrix gel using a pipette tip, collect the suspension, and transfer it to a centrifuge tube.
10. Place the organoids to be passaged on ice for 5-10 min.
11. Use a pipette tip to gently pipette and disrupt the matrix gel, repeating the process until the matrix gel is broken up, avoiding the formation of bubbles as much as possible.
12. Transfer the digested small intestinal mucosa to a centrifuge tube, add PBS, shake well, filter through a 70 μm cell strainer, centrifuge at 1000 rpm at room temperature for 5 min. Colon crypts do not need to be filtered.
13. Discard the supernatant, add culture medium to resuspend the pellet, count the crypts, with an optimal quantity of approximately 10 crypts/μL.
14. Add a certain amount of suspension to a centrifuge tube and centrifuge at room temperature for 5 min.
15. Add pre-chilled matrix gel mixture (matrix gel: culture medium = 2:1) to resuspend the pellet.
16. Passaging can be performed at a ratio of 1:1 to 3:1.
Freezing of Human Intestinal Organoids:
17. Place the organoids to be frozen on ice for 5-10 min.
18. Collect the organoids, discard the supernatant after centrifugation.
19. Add freezing medium to resuspend the organoids, aliquot into cryovials, and store at -80°C. After 24 hours, transfer to liquid nitrogen for long-term preservation.
The establishment of in vitro intestinal models using intestinal organoids greatly promotes research on the occurrence and development of intestinal diseases. It provides a new and important research platform and approach for high-throughput drug screening, new drug development, drug function testing, targeted therapy, personalized precision medicine, and regenerative medicine. The emergence of intestinal organoids opens up a new avenue for the research and treatment of intestinal diseases.
