How to Cultivate Human Brain Organoids

As an increasing number of neurological disorders remain unconquered, posing a threat to human health, understanding the full functionality of the brain and being able to treat various neurological diseases are the directions every neuroscientist is dedicated to. Due to practical and ethical constraints, acquiring experimental brain tissue is difficult, and traditionally, certain rodents are often used as model organisms for neuroscience research. However, the similarity in brain tissue structure and development between humans and rodents is not very high. Therefore, culturing brain organoids in vitro is a pressing issue that needs to be addressed.
Researchers can differentiate human pluripotent stem cells (hPSCs), including induced pluripotent stem cells (iPSCs) and embryonic stem cells (ES), to obtain neural cells and culture brain organoids in vitro to simulate the human environment. This is advantageous for researchers to develop therapeutic approaches to cure diseases.
Cultivation Protocol: Literature One
Research team developed a method to differentiate human pluripotent stem cells into neuronal cell layers expressing human midbrain characteristic markers. They reprogrammed patient's skin fibroblasts into iPSCs, then cultured them in a medium supplemented with FGF-basic, CHIR-99021, and MEK inhibitor (PD0325901).
Neuronal induction culture medium (DMEM/F12): Neurobasal, 1:100 N2, 1:50 B27 without vitamin A, 1% glutamine, 10 μM SB-431542, 200 ng/mL Noggin, 0.8 μM CHIR-99021, and 10 μM ROCK inhibitor Y-27632, 0.1% β-mercaptoethanol, 1 μg/mL heparin. Y-27632 was added for the first 48 hours, and the medium was changed on the second day.
Three days later, hMLOs began to squeeze out the neural ectoderm bud. At this point, the medium was completely removed, and 30 μL of reduced growth factor matrix gel was immediately added to each well using a pre-cooled 200 μL pipette tip. The midbrain organoids embedded in the matrix were placed in a 37°C incubator for 30 minutes to solidify the matrix.
Tissue growth induction culture medium contained neurobasal medium, 1:100 N2, 1:50 B27 without vitamin A, 1% glutamine, 0.1% β-mercaptoethanol, 2.5 μg/mL insulin, 200 ng/mL laminin, 100 ng/mL SHH-C25II, and 100 ng/mL FGF8.
Differentiation culture medium consisted of neurobasal medium, 1:100 N2, 1:50 B27 without vitamin A, 1% glutamine, 100 μM ascorbic acid, 125 μM DBcAMP, 0.1% β-mercaptoethanol, 10 ng/mL BDNF, and 10 ng/mL GDNF. Antibiotics were added to the medium to prevent potential bacterial contamination during long-term culture. The medium was changed every 3 days.
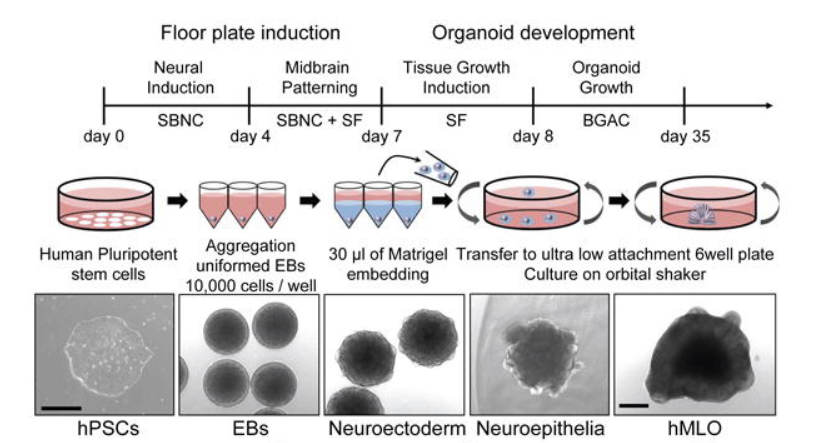
Figure 1. Typical Morphology of Cells at Each Stage of Human Midbrain Organoids (SBNC: SB-431542, CHIR-99021, Noggin) [1]
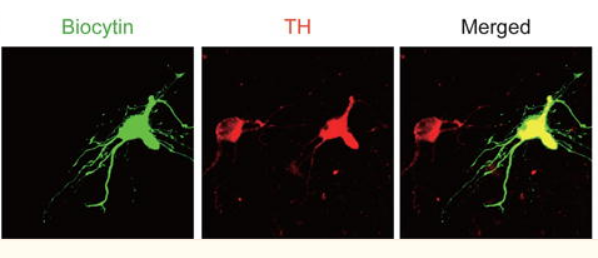
Figure 2. Neuronal Immunostaining [1]
Literature 2
Frontotemporal dementia is a highly debilitating neurological disorder characterized by behavioral, speech, and cognitive impairments. Mutations in the tau protein are known to be closely associated with this disease, but the specific mechanisms involved are not fully understood. Therefore, a research team utilized human-induced pluripotent stem cells (iPSCs) to construct in vitro brain organoid models of frontotemporal dementia with most of the damage, expressing mutant tau protein. This allowed for the elucidation of early molecular changes in neuronal damage, the explanation of pathogenic mechanisms, and the discovery of a drug, Apilimod, capable of inhibiting neuronal death.
Spheroid Formation Medium (SFM): Comprised of E8 medium and 10 mM ROCK inhibitor Y-27632.
Differentiation medium: E6 medium supplemented with 2.5 mM Dorsomorphin, 10 mM SB-431542, and 2.5 mM XAV-939; 1 mL of medium added per spheroid. Spheroids gently mixed and incubated at 37°C with 5% carbon dioxide for 48 hours.
Every day, gently aspirate the medium from the culture dish and replace it with differentiation medium, achieving 65% medium exchange from day 2 to day 5. On day 6, switch the medium to Neural Medium (NM), consisting of neural basal medium, B27 without vitamin A, glutamate, and anti-A, supplemented with 20 ng/mL EGF + 20 ng/mL FGF2.
Starting from day 25, supplement NM with 20 ng/mL BDNF and 20 ng/mL NT3 (medium C), replenishing 65% of the medium every other day. From day 43 onwards, replace the medium every 3-4 days without adding growth factors, changing 15-20 mL of medium per culture dish, and replacing 75% of the medium. Throughout the culture period, fused organoids were dissected apart using a disposable surgical blade. In the experiments depicted in the figure below, induced pluripotent stem cells were grown and patterned.
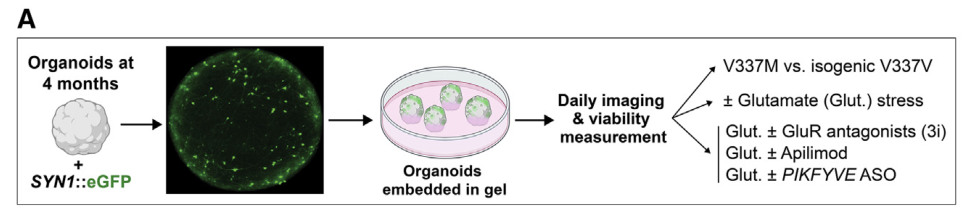
Figure 3. Longitudinal Axis of Neuronal Survival in Brain Organoids [2]
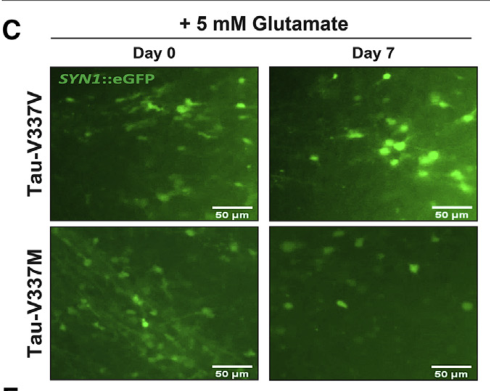
Figure 4. Images of tau-V337M and wild-type V337V organoids treated with 5 mM glutamate [2]
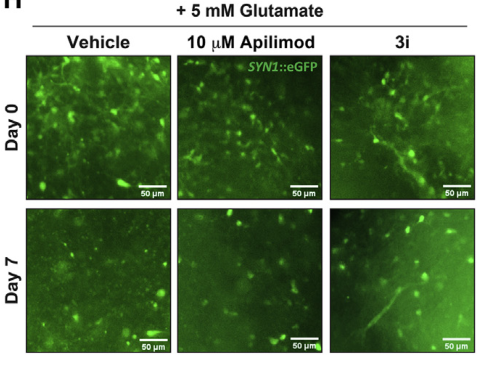
Figure 5. Images of tau-V337M organoids treated with 5 mM glutamate salt and DMSO [2]
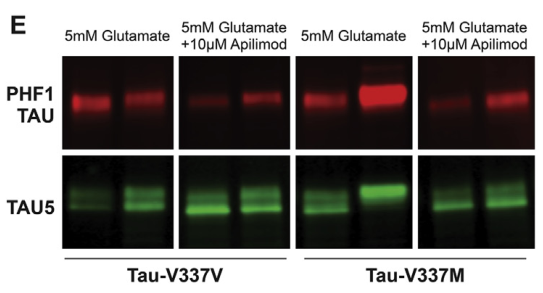
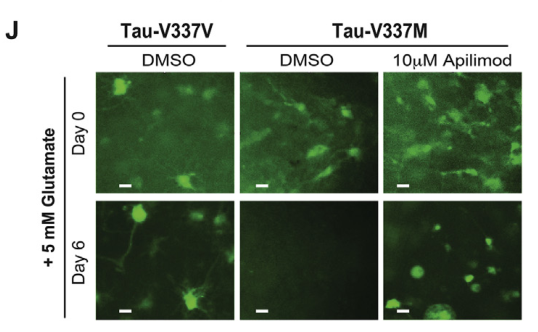
Figure 6. Reversal of Glutamate Excitotoxicity Sensitivity in tau-V337M Organoids by Apilimod [2]
Literature 3
Human brain development exhibits several unique aspects, such as increased complexity and expanded neuronal outputs, which have been demonstrated to be challenging to study in model organisms. Therefore, in vitro methods that mimic human brain development and diseases have become a hot research area.
Here, a research team has established a brain organoid culture model that mimics endogenous developmental programs. This model can be cultured in a standard tissue culture incubator and develop cortical regions, forebrain, and even retinal tissues within 1-2 months. This approach can be applied to developmental studies as well as research on various human brain diseases.
Furthermore, since organoids can be maintained in culture for over a year, they also have the potential to simulate later events such as neuronal maturation and survival.
Human pluripotent stem cells generate brain organoids: by adding L-alanyl-L-glutamine solution, ROCK inhibitor Y-27632, N-2 supplement, B27 without vitamin A, B27+vitA supplement, Penicillin-Streptomycin, bFGF, etc. [3]
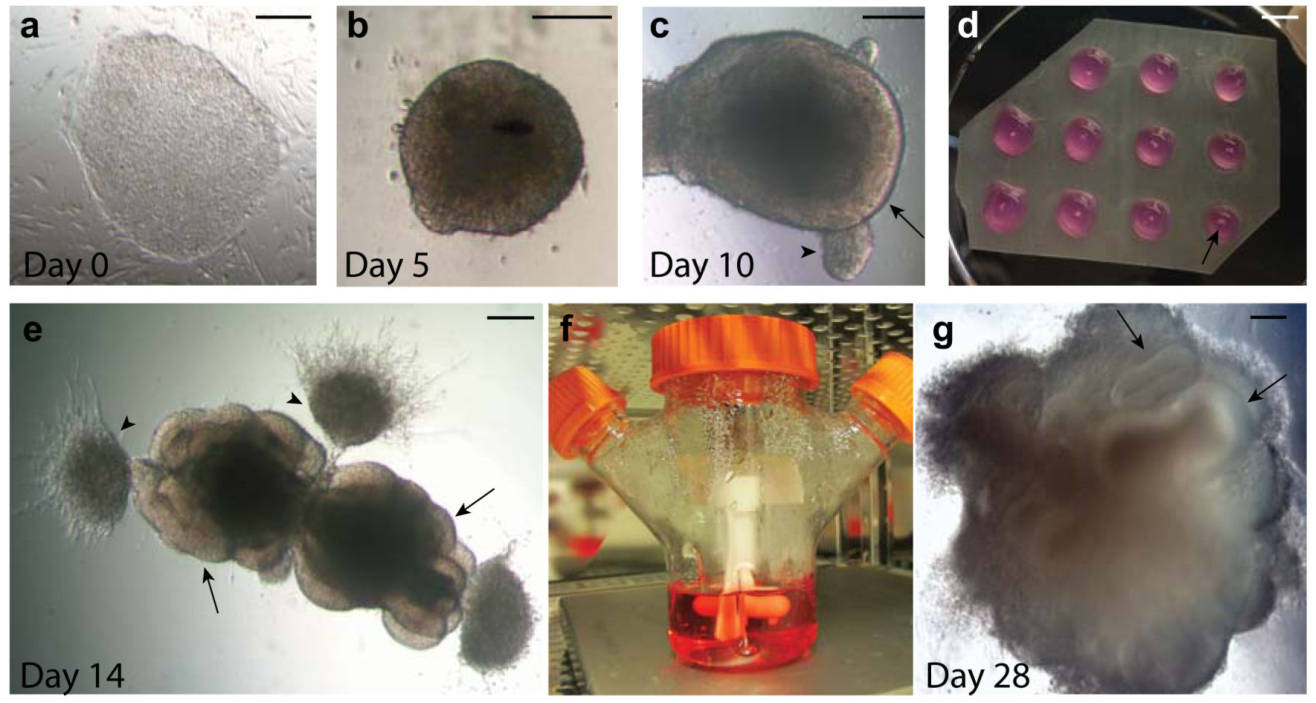
Figure 7. Progression of Human PSC-Derived Brain Organoid Development [3]
Summary of Compounds Added to Brain Organoid Cultures
|
Product Name |
Catalog Number |
Specification |
|
Recombinant Human BDNF Protein, Flag tag |
C230262 |
5/20/100/500 μg/1 mg |
