Arcegel Matrix Gel Application - Construction of Mouse Intestinal Organoids

Organoids are 3D cell cultures cultivated in vitro using stem cells, progenitor cells, tumor cells, etc., and exhibit spatially organized structures resembling certain tissues. In 2021, organoid technology was listed as a key research and development focus in China's "14th Five-Year Plan." As disease models, organoids provide a more physiologically relevant environment compared to traditional 2D disease models, facilitating the elucidation of disease progression, homeostasis, pathogenesis, and other aspects. They are expected to play a significant role in various fields such as cell therapy, drug development, genetic engineering, immunology, tissue regeneration, and more.
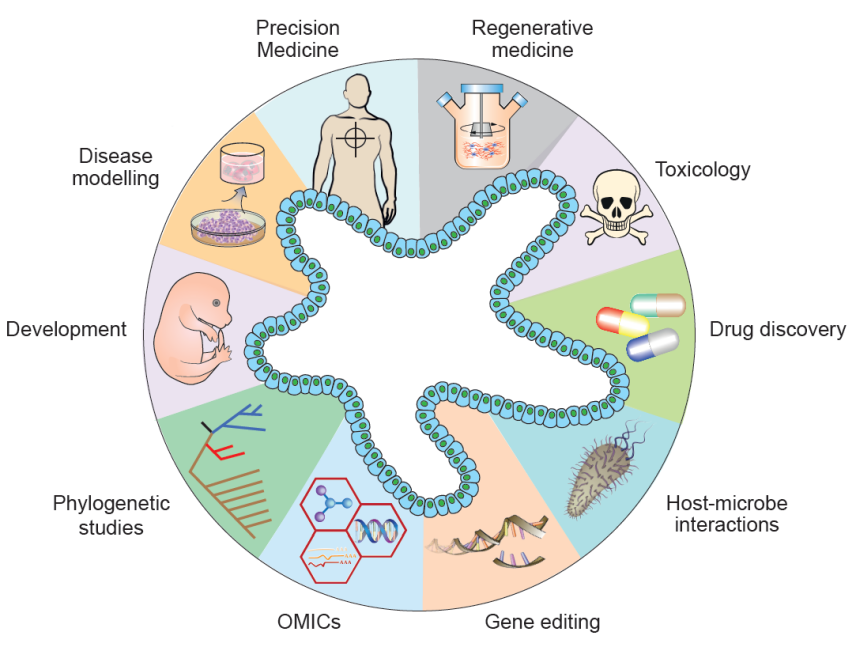
Figure: Application Directions of Organoids
Organoids are primarily constructed from adult stem cells or tumor samples under suitable culture conditions. The overall culture system of organoids includes tissue digestion, washing, processing, medium preparation, mixing of matrix gel with cells, culture, observation, and other steps. Among these, the mixing of cell suspension with extracellular matrix (matrix gel) is one of the key steps, providing a 3D growth substrate for organoids.
The main components of matrix gel include laminin, type IV collagen, heparan sulfate proteoglycans (HSPG), nidogen, and other growth factors such as TGF-beta, EGF, IGF, FGF, tissue plasminogen activator, and other growth factors present in EHS tumors. It provides support, tensile strength, and scaffold support for tissues and cells. Arcegel Matrix Gel developed and produced by Arcegen Biology is free from LDEV (lactate dehydrogenase-elevating virus), has an ultra-low endotoxin content, and has been thoroughly tested for mycoplasma to ensure no contamination, including different types of matrix gels such as basic concentration, high concentration, and low growth factor concentration.
Application Direction
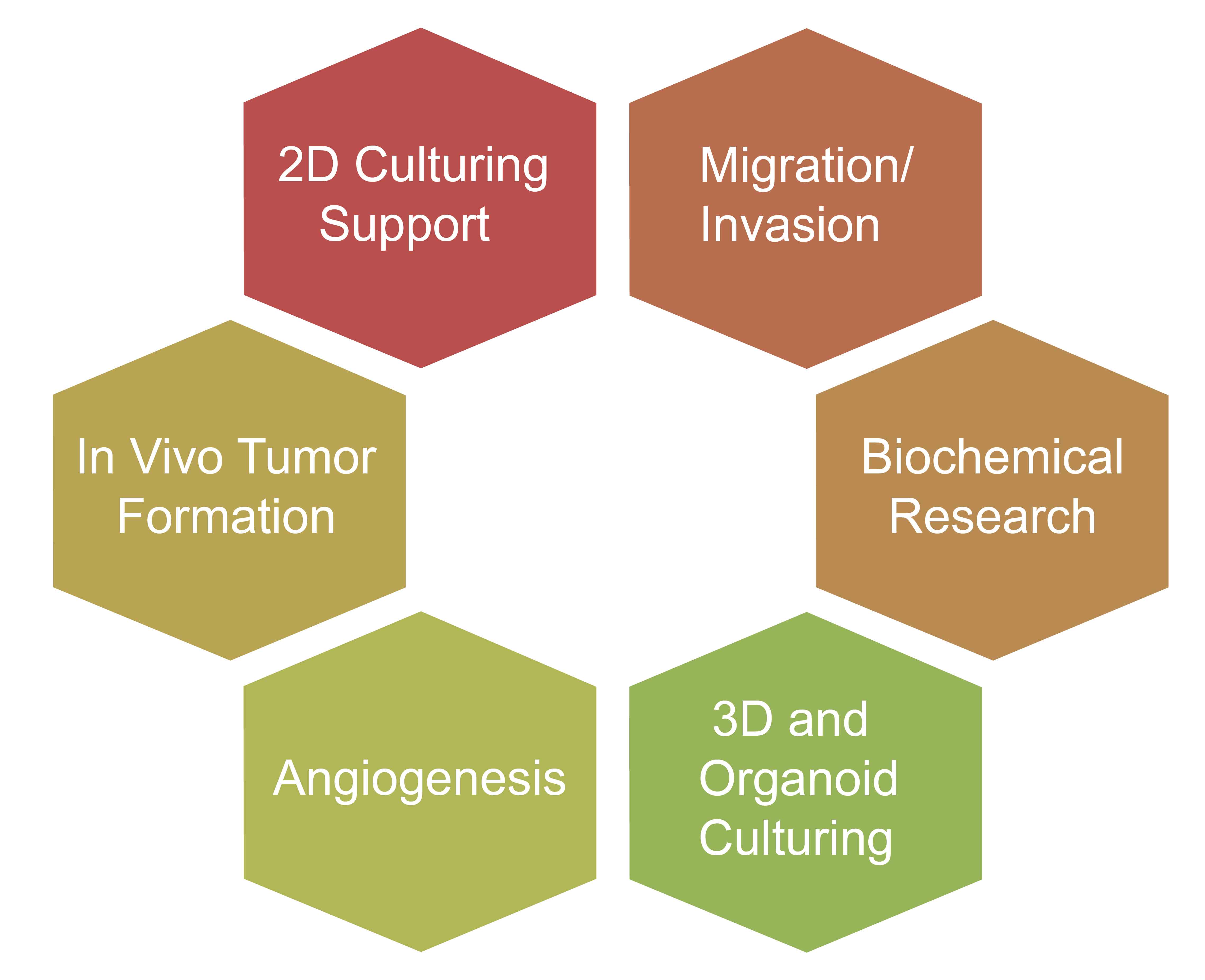
Mouse Small Intestine Organoid Construction
Experimental Procedure
1. Sample Preparation: Mice were euthanized by cervical dislocation, and the surface was sterilized by spraying with alcohol. Under aseptic conditions, the intestine tissue located near the proximal end of the stomach, 3~15cm in length, was excised using forceps, carefully removing the intestinal mesentery and fat. The excised tissue was placed in DPBS solution pre-cooled to 4°C containing 1% antibiotics.
2. Sample Washing: The intestine was rinsed 2-3 times using a syringe, and then the intestine tube was carefully opened with surgical scissors, exposing the lumen with the mucosal side facing up. Using a surgical blade, the intestinal villi were gently scraped off the mucosal surface until the tissue became transparent. The tissue was then washed 2~3 times in a new DPBS-containing culture dish.
3. Initial Sample Processing: The washed small intestine tissue was cut into small pieces approximately 2mm in width and transferred to a new 50ml centrifuge tube. The tissue was gently washed 3-5 times with DPBS to remove intestinal villus cells and floating fat tissue.
4. Sample Digestion: The washed small intestine tissue fragments were digested in pre-cooled DPBS containing 3-5mM EDTA, with agitation at 4°C for approximately 30 minutes. The centrifuge tube was gently shaken every 10 minutes during the incubation period.
5. After digestion, the supernatant containing EDTA digestion solution was discarded, and the tissue was gently washed 2~3 times with fresh DPBS buffer to remove residual EDTA.
6. 10~15ml pre-cooled DPBS containing 0.1% BSA was added to the small intestine tissue fragments. The tissue fragments were repeatedly pipetted and suspended to separate crypts from the basal layer. A small amount of suspension was examined under a microscope, and when a large number of crypt-like structures were observed, the pipetting was stopped. The tissue suspension after pipetting was filtered through a 70μm filter and the filtered tissue suspension was collected.
7. Repeat steps 5-6 twice, centrifuge at 1500rpm, 4°C for 3 minutes.
8. Formation of Mixture: Re-suspend the crypt tissue precipitate in Arcegel matrix gel. Every 10μL of matrix gel suspension contains 200~600 crypts. After resuspension, the mixture was placed on ice and processed as soon as possible to avoid gel formation. Note: The dilution ratio of the matrix gel should be ≥50% to ensure the stability of the Arcegel matrix gel structure during the culture process.
9. Plant the mixed suspension in the center of the bottom of a 24-well plate, approximately 30~50μL per well, avoiding contact between the suspension and the side walls of the plate.
10. Place the cultured plate in a 37°C carbon dioxide incubator and incubate for approximately 30 minutes until the matrix gel solidifies.
11. Once the Arcegel matrix gel is completely solidified, slowly add pre-prepared intestinal organoid culture medium along the well walls, 800μL per well.
12. Place the 24-well plate in a 37°C carbon dioxide incubator. Change the fresh culture medium every 3 days and monitor the growth status of the organoids. Generally, mouse small intestine organoids form within 5~7 days.
Experimental Results
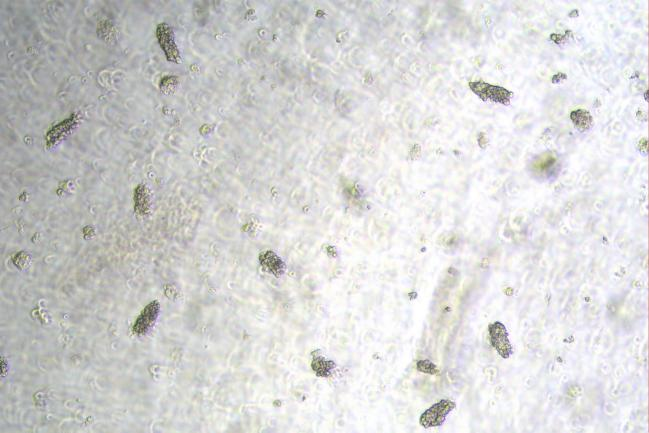
Day0
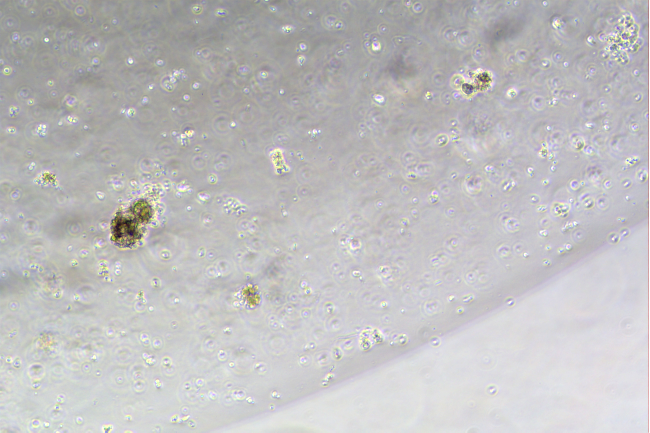
Day3
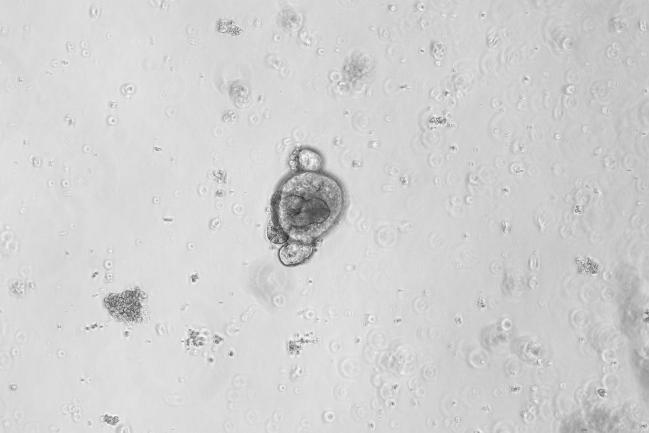
Day5
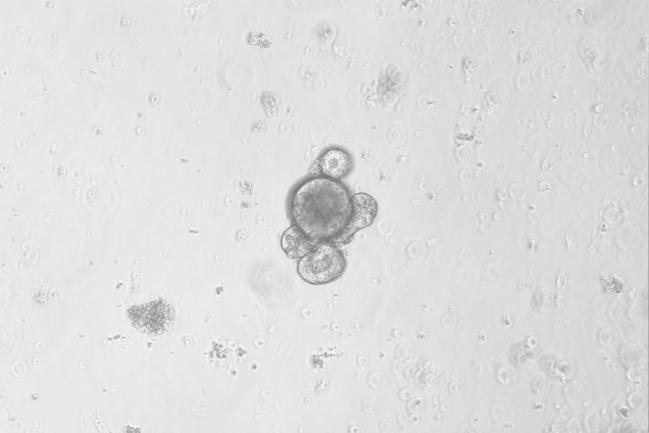
Day7
Figure: In Vitro Culture Results of Mouse Small Intestine Organoids
FAQ
1. What digestive solution should be used for tissue digestion and how long should digestion last?
For tissue digestion, EDTA or collagenase can be used. EDTA is suitable for hollow organs like the small intestine and stomach, while collagenase, which comes in various types and can be combined with DNAase, is suitable for a wider range of tissue types. The duration of digestion varies significantly, ranging from several minutes to several hours depending on the tissue type.
2. What precautions should be taken when handling the matrix gel?
All operations should be conducted under aseptic conditions, and pre-cooled pipettes should be used to ensure that the matrix gel is in a homogeneous state.
3. How should the matrix gel be aliquoted, frozen, and used?
The thawed Arcegel matrix gel can be aliquoted into multiple small tubes, all of which should be pre-cooled. The aliquots should be quickly frozen and stored to avoid repeated freeze-thaw cycles.
4. What are the volumes of matrix gel and culture medium needed for culturing organoids in different well plates?
For culturing organoids, a 24-well plate is commonly used, with 50μl of matrix gel per well followed by the addition of 500-800μl of culture medium to cover the gel droplets. In a 96-well plate, 10μl of matrix gel per well is used, followed by the addition of 200μl of culture medium. For a 6-well plate, multiple 50μl gel droplets can be seeded per well, followed by the addition of 2-3ml of culture medium to cover the gel droplets.
Related products
|
Product Type |
Product Name |
Catalog Number |
Specifications |
|
Basic concentration |
C231001 |
5/10 mL |
|
|
C231002 |
5/10 mL |
||
|
Low growth factor |
C231003 |
5/10 mL |
|
|
C231004 |
5/10 mL |
||
|
High concentration |
C231005 |
5/10 mL |
|
|
Arcegel Matrix High Concentration, Phenol Red-Free, LDEV-Free |
C231006 |
5/10 mL |
|
|
C231007 |
5/10 mL |
||
|
Stem cell-specific |
C231008 |
5/10 mL |
|
|
Organoid-specific |
Arcegel Matrix for Organoid culture, Phenol Red-Free, LDEV-Free |
C231009 |
5/10 mL |
